| Hynobius dunni | |||||||||||
| Oita Salamander | |||||||||||

|
|
||||||||||
Description
This species has a dorsal coloration consisting of round black dots over a gray-green background color. Some animals (even of the same brood) entirely lack these black spots, and the spots tend to fade as the animals get older, with older adults having a more uniform grey-green coloration. Like most Hynobius, juveniles also have blue iridescent coloration. These blue highlights may even be present into the second year of life, when they have almost reached their adult length. The blue coloration seems to be more pronounced when the animals are kept in a wetter environment. Most animals have 12 costal grooves, but animals with only 11 have been documented. Total length averages 10 to 16 cm (3.9 - 6.3 in), while snout to vent length averages approximately 6 to 8 cm (2.4 - 3.1 in).
The animals cannot be sexed by any external indicators, except during the reproductive period. During the breeding season, the tail of the animals becomes entirely yellow, and males develop an enlarged head, high tail fin, and light-colored throat.
According to the literature, Hynobius dunni can live up to 16 years. Currently, my adult group is 7 years of age. In my opinion, this easily-maintained salamander has a good chance of becoming a widespread terrarium animal. Since 1998, my animals have reproduced every year, and in my opinion they are extremely hardy compared to other salamanders and newts.
 Adult H. dunni (7-8 years old). |
 Young adult H. dunni (3-5 years old) showing black dots and blue coloration, which will fade with age. |
Natural Range and Habitat
Hynobius dunni only occurs on the Japanese Island groups of Shikoku (Kouchi-ken) and Kyushu (Ohita-ken, Miyazaki-ken, Kumamoto-ken). Hynobius species can be divided into two ecotypes, the pond ecotype and stream ecotype. H. dunni is an example of the pond ecotype.
Hynobius dunni lives in secondary forest and bamboo woods on low hills. Reproduction occurs in pools and swamps filled with dead leaves and fallen twigs, or in the small pools and slowly flowing ditches alongside rice fields. Reproduction begins in December and can extend through the end of April, with a peak generally occurring in February (depending on the weather). Outside this period, the animals can be found under stones and fallen leaves (translation from Sengoku, 1979).
No exact temperature measurements have been published for the habitats of H. dunni. However, air temperatures in Oita Prefecture, which is the collection locale of my animals, range from average January lows of 1-7°C (34-45°F) to average August highs of 24-27°C (75-81°F). Like most salamanders, H. dunni is probably protected from these temperature extremes by its microenvironment.
 Young adult H. dunni. Although sexually mature, this male retains some juvenile coloration. |
 Adult H. dunni. |
Conservation Status
Due to the destruction of their breeding habitats, mainly for the construction of new houses, this species is beginning to become threatened. At the end of 1999, H. dunni, together with some other Hynobius species, was placed on the Japanese Red List with the status of "vulnerable" (Ikeda and Kawamura, personal communication). Since this species has been put on the Japanese Amphibian Red List, and the fact that this animal is rarely kept in captivity, I started a studbook. Through breeding and rearing with an original group of 10 young animals, the studbook population contained over 300 animals, as of 2001.
Housing
A tank for any pond-type Hynobius should have both a land and a water section. Outside of the breeding season, the animals will want to be on land, but I observe them entering the water at twilight in search of food. During the breeding period, they need a substantial water area, especially the males, which have quite a long aquatic phase. Two separate tanks can also be used, one with water (and some land) for reproduction, and one with just land and leaf litter, moss, and wood from the forest floor for outside the breeding period. While I started out working with the one-tank system, I now prefer to work with the double-tank system. The advantages are that it is closer to the natural life cycle of the salamanders, and that they seem to put on more weight during the pure land phase. The disadvantage is that you need more room. I describe below the methods I used for over 8 years keeping them with the single-tank system. Observations by other people in the studbook seem to confirm that this method works without problems.
 Construction of a setup for H. dunni. Water will be added. |
 Setup for H. dunni. The animals like to stay hidden and wet. |
I use a setup that includes both water and land. My breeding group (8 animals) is housed in a paludarium 140 x 50 cm (4 x 1.5 feet). The bottom substrate consists of washed sand. Water depth is approximately 10 to 12 cm (4-5 in) at the deepest part. Two-thirds of the tank consists of an island of broken yellow construction bricks, which are light-weight and have many long aeration holes. The salamanders like to use those holes for shelter. On top of these bricks comes a thick carpet of moss, approx 10 cm (4 in) thick. Through capillary action, water is sucked up by the moss to the upper areas of the bricks. This thick plant carpet also plays an important role in temperature maintenance by shading the water beneath. On top of the moss are placed pieces of bark.This whole setup acts like a mini-biotope: some self-sustaining prey animals like woodlice may even breed in the tank.
The tank is placed in a frost-free shed, which is located under a tree (for shade in summer). A Plexiglass roof panel exposes the animals to the natural photo-period, which is necessary for reproduction. No supplementary lighting is given. During the winter, the temperature drops to just around freezing. On rare occasions a little bit of frost might set in.
Overall, the animals have a secretive lifestyle. They are active during periods of cool temperatures from 14° to 16°C (57-61°F), which occur mainly during spring and autumn. During these periods, one can find them in the water. During winter and summer, they remain on land, hiding in the previously mentioned holes in the bricks. They clearly seem to prefer those openings that have a gradual slope upwards out of the water. Here they stay very wet, even with some part of their body in the water. This level of humidity is also a requirement for H. leechi quelpartensis (Ad Bouwman, personal communication). In my experience, there exists an inverse relationship between age and the amount of time spent in the water. While younger animals seem to visit the water areas even outside of the breeding season, their presence decreases significantly with age (all other conditions being equal).
Feeding
These salamanders are not picky eaters. They willingly accept earthworms, miniature mealworms (buffaloworms; Alphitobius diaperinus), fly maggots, mosquito larvae, woodlice, millipedes, and other insects that can be found while gardening. The addition of prey animals to the tank during the terrestrial winter period quickly results in plump animals. They eat during this period, but do not consume much energy. In contrast to other salamanders, none of my Hynobius salamanders seem to eat slugs.
 Light throat coloration occurs on males during breeding season. |
 Male marking a branch. Note enlarged head typical of males during breeding season. |
Breeding
In order to create possible spawning places and to facilitate observation, the water area is filled with dead branches. They are best positioned in such a way that for some part of their length they run horizontally along the water surface. Thickness is important, since the branch will need to carry the weight of several animals. Sato used a branch of 1.3 cm (0.5 in) for his H. retardatus study (Sato, 1992). Both Hynobius dunni and H. retardatus seem to prefer such a robust branch. The female needs this to deposit her egg masses, while it also plays an important role in the breeding strategy of the male.
During reproduction, the males will - as with many species - enter the water first. These first migrations to the water have happened at a temperature of 5°C (41°F) and higher, which occurs between January and March, depending on the weather. Males may remain aquatic until April or May. During the reproductive period, the head of the males becomes quite broad and the tail develops a higher fin. With H. dunni, I have never observed any territorial behavior among the males. The males seemingly crawl quite randomly over the bottom and the twigs, without any sign of chasing the others. This tolerance changes dramatically in the presence of a gravid female. The males will search for a possible spawning place and will cautiously follow any female approaching such a place, especially if she starts climbing on it.
 Above: Female finding an egg-laying site. Right: Egg-laying, with males competing to fertilize the eggs. |

|
In the presence of a female, courtship begins. At first all the males walk around on the bottom of the tank (wandering phase) and are quite interested in the branches. From time to time, they start to climb the branches (climbing phase). Most of their interest is clearly focused on those branches placed horizontally just under the water surface. Males may begin tail fanning and inflate their already-thickened throat. I call this behaviour the attraction phase. A male may then rub his cloaca on one particular spot where he has been tail fanning. I call this behaviour the marking phase. The whole cycle (wandering, climbing, attraction, and marking phases) may be repeated many times without success. About every 10 to 15 minutes the attraction phase is displayed. When females come near, they attract the attention of the males, who, in their excited behavior, may accidentally push the female off the branch. Eventually, she will lay a pair of egg sacs, often near the point marked by the male during the marking phase.
When a female makes a gesture as if she intends to spawn, the males will get closer to her. Due to the tiny size of the eggsac initially, it is important to be there first: the first male has the advantage that he can use his body mass to monopolize the eggsac for a while. All of the males will try to climb on the sac in order to try and fertilize it. This may lead to some commotion and a large clump of males nervously trying to climb on each other and the eggs; this is referred to as a mating ball.
Like with all Hynobius, the female lays an eggsac on branches or twigs. The sac consists of 2 separate banana-shaped sacs that the males will try to fertilize externally. At deposition, these eggsacs are still small. They absorb water during the first days and actually become larger than the female. According to the literature, the egg masses of Hynobius dunni typically measure between 15 and 30 cm (6-12 inches). The egg masses from my animals never came near this maximum length. Each separate mass may contain 80 to 140 eggs. Mashiba reports that egg deposition happens at 9°C (48°F)(Mashiba, 1969). After a few days, the outside layer of the eggsacs becomes a quite tough and elastic. This might deliver a certain degree of protection against eventual dehydration and certain predators, such as Planaria.

Newly-laid egg sac. It will expand with water during the first week after laying.
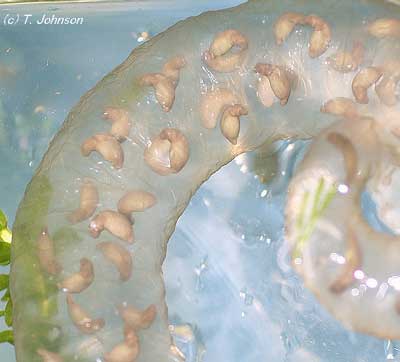
Developing egg sac.
 Developing egg sac. |
 Egg sac near hatching. |
Raising larvae and juveniles
As the larvae near hatching, the outer membrane of the eggsac seems to become less tough. Inside the eggsacs, the larvae hatch from their individual eggs, then they appear to be trapped inside the egg sac. After a while, the rear end of the eggsac opens, and the larvae swim or fall out. In nature, the larvae probably fall down through the rear end of the eggsac. In captivity, the egg sac has often been torn in other places. In the past, I have tried opening up eggsacs with larvae that seemed to be trapped inside, but the majority of these larvae died. So it may be important to leave the eggsacs unharmed; however, I have not done any hard tests to actually confirm this.
Egg sacs hatch in approximately 5-7 weeks. Shortly after hatching, these salamanders develop a pair of well-developed balancers. According to the literature, they feed on aquatic insect larvae and freshwater shrimp (Gammarus spp). In populations with higher densities, cannibalism occurs. The larvae grow to a length of 4 cm (1.5 in) by July.
In captivity, the larvae can initially be fed daphnia, and later mosquito larvae and white worms. Another preferred food source is tubifex worms, which are readily accepted even in the first weeks after hatching. These larvae often have a gigantic appetite, which goes hand in hand with aggression. Larvae of merely 4 cm (1.5 in) are able to eat large fly maggots of approx 1 cm (0.4 in), which they digest without any apparent trouble. Once the larvae are 3 cm (1 in), they do not hesitate to chase their eventual prey around throughout the tank. In my experience, cannibalism appears to be very rare.
 H. dunni larvae. |
 |
The larvae have a benthic life-style: they are bottom dwellers. They are usually observed crawling along on the bottom, constantly searching for food. When a cloud of daphnia is introduced, they start to stratify, gliding through the water while chasing and feeding on the daphnia. Under crowded conditions, Hynobius dunni larvae come up frequently gulping for air. High larval densities, warm temperature, and lack of water changes are probably the main causes. However, this behavior does not result in death.
In nature, metamorphosis starts in August to September, and occasionally some overwinter as larvae. In captivity, the length of larval development varies widely, with the larvae usually metamorphosing in late spring through late summer. Keeping them cooler (below 20°C, preferably below 16°C) delays metamorphosis and allows them to reach a greater length before metamorphosis. Even before metamorphosis begins, one can clearly recognize the usual Hynobius head shape on these late-stage larvae: a short mouth with eyes positioned close to the tip of the snout. At the first signs of metamorphosis, the costal grooves begin to appear. Shortly thereafter, the fins begin to shrink and disappear, and lastly the gills do the same. Some of the juveniles venture onto land while small gill buds are still present.
Each year, 2 distinct color morphs develop among the juveniles. I observed a group of tinier black-colored animals and a group of larger yellow-brown animals. This latter group also had round black dots and thus already showed the adult coloration. Within both the groups, the body had lateral blue iridescent points, sometimes making the whole flank look blue. A few months later however, the dark animals also develop into the light brown adult color pattern. As they age, the blue iridescent spots fade, only to re-emerge - to a lesser extent - during the reproductive period.
The raising of the juvenile animals happens in a miniature version of the parental tank. For a detailed description, see Wallays (2000). Since this tank is covered by a glass plate with a fluorescent lamp on top, the air temperatures can occasionally get up to 27°C (80°F), especially during the summer months. Until now, this has not been a problem, probably because of the presence of cooler hideouts. In contrast to their sensitivity during of the early larval phase (<3 cm; 1 in) young Hynobius dunni seem to be quite tolerant of temperature and water quality. During the winter, their growth rate can be strongly enhanced by keeping them at temperatures of 14 to 20°C (57-68°F) and feeding them daily.
 Newly-metamorphosed H. dunni. |
 Juvenile H. dunni. |
 Juvenile H. dunni. |
 Juvenile H. dunni. |
Other Resources
Hynobius dunni account on AmphibiaWeb
"Hynobius: literature study with some personal observations" (Available from the author)
References
Bouwman, A. 1995. Temperatuur en kweeksucces met de Hoektandsalamander van Leech (Hynobius Leechi). Lacerta 53(3): 91-95.
Bouwman, Ad, personal communication.
Ikeda, Jun, personal communication.
Japan Meteorological Agency. http://www.data.kishou.go.jp/etrn/index.html. Accessed 11/2004.
Kawamura, Mamuro, personal communication.
Kusano, T. 1981. Growth and survival rate of the larvae of Hynobius nebulosus tokyoensis TAGO (Amphibia, Hynobiidae), Research on Population Ecology 2: 360-378.
Kusano, T. 1985. Size related cannibalism among larval Hynobius nebulosus. Copeia 2: 472-476.
Mashiba, S. 1969. Ecology of Hynobius dunni - chiefly its breeding activity. Saishu to Shiiku (Collecting and Breeding) 31(5): 122-135 (Japan).
Sato, T. 1992. Reproductive behavior in the Japanese salamander Hynobius retardatus. Japanese Journal of Herpetology 14(4): 184-190.
Sengoku (ed.) 1979. Amphibians and Reptiles in color (Japan).
Thorn, R. 1991. Observations et notes sur diverses espéces de Salamandres (Amphibia, Caudata) Bull. Soc. Nat. Luxemb. (92): 79-83.
Usuda, H. 1995. Waving behavior and its effect on the reproductive behavior of Hynobius nigrescens. Japanese Journal of Herpetology 16(1): 19-24.
Wallays, H. 2000. Raising metamorphosed juveniles. Indiana University Axolotl Newsletter 28: 10-17.
©2005 Henk Wallays. Posted April 2005.
